PRODUCT INFORMATION
FEATURES AND BENEFITS
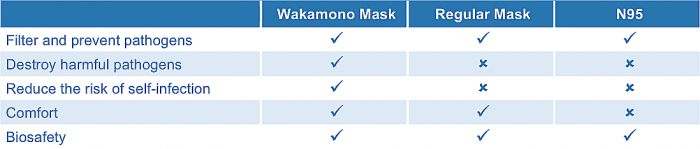
Wakamono 4-layer surgical mask is a single use, disposable surgical mask that covers the nose and mouth of the wearers to protect from the transfer of microorganisms. body fluid, and particulates. The mask can provide its user the maximum level of protection with dual mechanism of filtration and inactivation as well as high breathability and comfort. The elastic ear-loops to secure the mask to the user’s face, and has a plastic strip positioned above the nose for a tighter seal around the nose and face.

CONSTRUCTION AND DESIGN
The mask is constructed with 4-layer non-woven fabric and each layer has different characteristics.
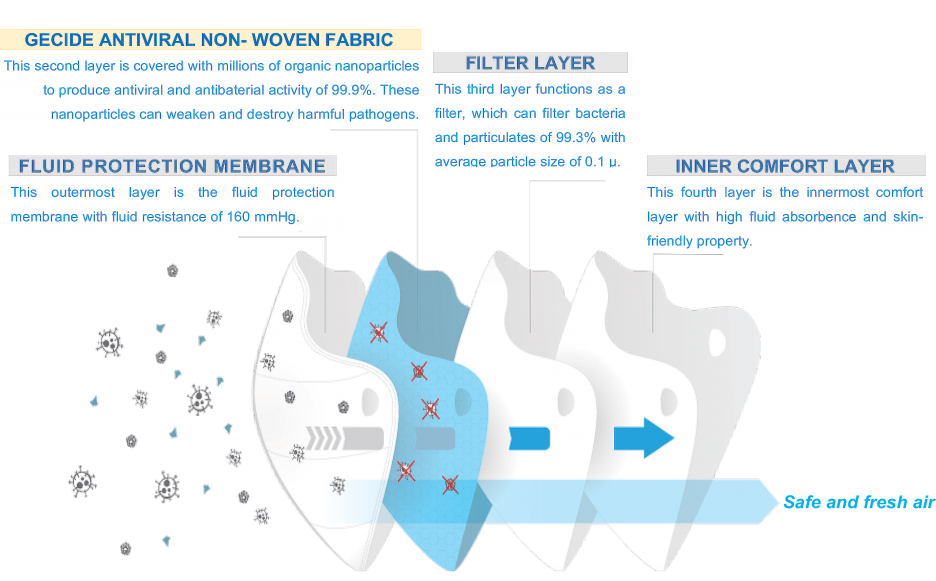
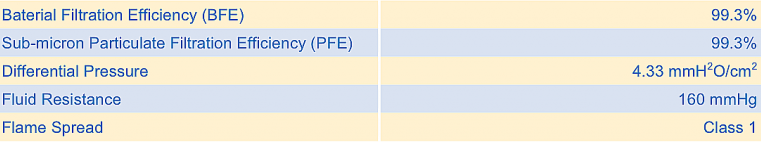
The outermost layer is the fluid protection membrane with fluid resistance of 160 mmHg. The second layer is the GECIDE antiviral non-woven fabric layer which is treated with nano particles to produce antiviral and antibaterial activity of 99.9%. The third layer functions as a filter, which can filter bacteria and particulates of 99.3% with average particle size of 0.1 µ. The fourth layer is the innermost comfort layer with high fluid absorbence and skin-friendly property. In addition to that, the mask also achieves a high breathability with the differential pressure of 4.33 mmH²0/cm² . The mask performance was certified by TUV SUD laboratory (Germany).
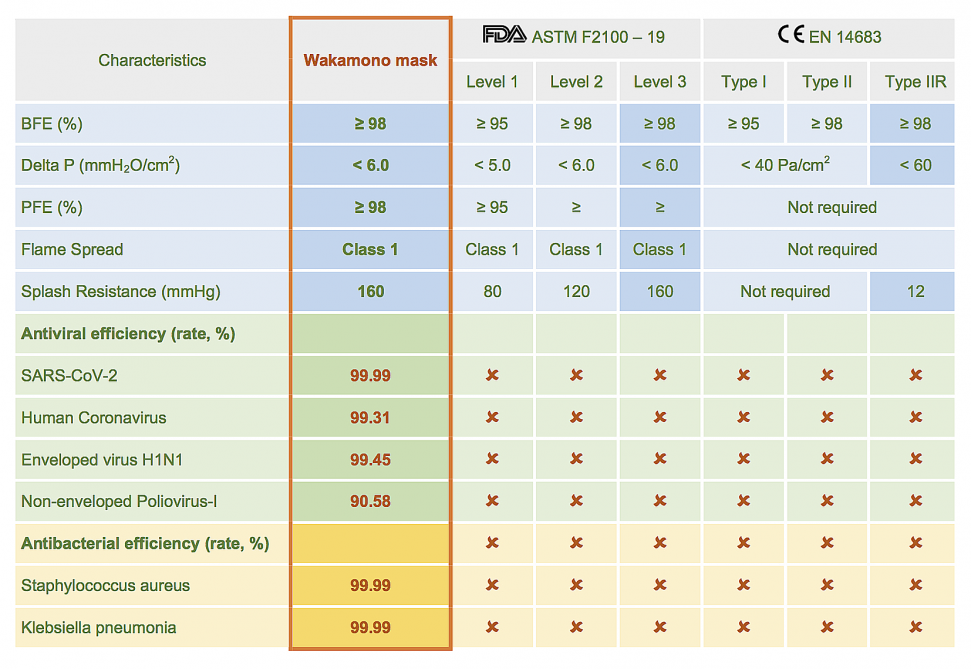
Wakamono surgical mask meets both U.S ASTM Level 3 and CE EN 14683 Type IIR.
MECHANISM OF ACTION OF ANTIVIRAL AND ANTIBACTERIAL ACTIVITY
The outstanding feature of Wakamono surgical masks is an antiviral and antibacterial property with the application of Micellium Nanotechnology. This is used to treat the Gecide layer and is then integrated into the second layer of the mask. The bionano particles in Gecide layer create coatings that can hinder bacterial and viral adherence and proliferation and, thus, inactivate the virus and bateria and prevent their spread. Unlike metal nanoparticles such as silver or copper — which are possible toxic¹ — the bionano particles such as micelium is the most natural way to kill bacteria and viruses. They are friendly and safe for humans and the environment according to most nanotechnology literatures².
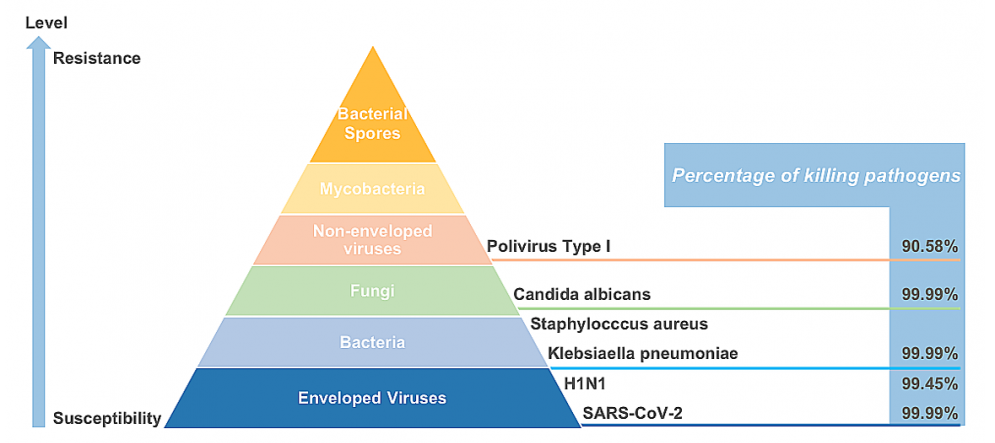
Eurofins Laboratory (U.S.) test results demonstrated that the Wakamono surgical mask with Gecide layer inactivates 99.99% of SARS-CoV-2 within 60 seconds of contact and maintained effectiveness up to 60 minutes. TUV SUD Laboratory (Germany) also confirmed the Gecide layer can reduce Human Coronavirus up to 99.9%³. Besides, the Gecide layer can also destroy 99% of H1N1 Influenza A virus (enveloped virus) and 90% of Poliovirus-I (non-enveloped virus). This property was tested by Guangdong Detection Center of Microbiology (China).
Antibacterial activity of Gecide layer against microorganisms such as Staphylococcus aureus, Pseudomonas aeruginosa, Candida albicans, Escherichia coli, Salmonella enterica subsp. eneterica serovar Typhimurlum was confirmed by Analytical Laboratory of Singapore. The test results showed Gedice layer destroys 99.9% of microorganisms within 60 seconds of contact. Bureau Veritas Laboratory (Cadana) confirmed the duration of microorganism growth inhibitory of Gecide layer lasts up to 48 hours when compared Gecide mixture with other control solutions including Ethanol 50%, Ethanol 70% and Chloramine 0.25%.
With the unique antiviral and antibactial property of the mask, Wakamono is applying for an OUK code, learn more at FDA U.S Food and Drug Administration
BIOSAFETY
Wakamono surgical mask is biocompatible. The Gecide Fabric layer of the mask is composed of organic and natural components. Its biosafety was certified by Pacific BioLabs (U.S.) and in accordance to ANSI/AAMI/ISO 10993-5:2009. Additionally, the nose clip of the mask is made of plastic instead of aluminum to achieve best fit as well as non-toxicity for users.
CERTIFICATE & AWARD
For more database, please enter the FDA registration no.: 3017461158 at FDA U.S Food and Drug Administration

As a science-led global Nano biotechnology company, Wakamono is selected as abright nominee for Global Business Excellence Awards of the year 2020 in the category Outstanding Innovation during COVID-19.

WORLDWIDE DISTRIBUTION
Wakamono mask is trusted and distributed to many countries around the world.
References:





